Introduction: Isatuximab (Isa), a humanized monoclonal antibody directed against the surface antigen CD38, has been available in Italy since October 2021 for the treatment of relapsed or refractory Multiple Myeloma (RRMM) in combination with Pomalidomide (P) and Dexamethasone (d) (IsaPd) after two lines of therapy with lenalidomide and a proteosome inhibitor, and from April 2022 in combination with carfilzomib (K) after one line of treatment. Patients eligible for these therapies must not be refractory to Daratumumab. Here, we report preliminary efficacy and safety data for Isatuximab in a Sardinian study of RRMM patients treated with IsaPd and IsaKda as salvage therapy outside of controlled clinical trials. The primary objective of this study was to evaluate the toxicity profile and quality of response to Isatuximab-based regimens administered as salvage therapy in a real-world setting.
Methods: The 2 cohorts included 27 patients with RRMM from 4 Sardinian centers who received at least one cycle of IsaPd (16 patients) or IsaKd (11 patients) as salvage therapy between January 2022 and March 2023. Patients received Isa 10 mg/kg IV weekly for the first 4 weeks (wks), then every 2 weeks in combination with Pomalidomide and Dexamethasone (4mg PO days 1-21; 40mg [20mg at > 75 years] PO or IV weekly) every 28 days for the IsaPd regimen. The IsaKd regimen consisted of administration of 10 mg/kg Isatuximab intravenously once weekly in the first cycle, then once every 2 weeks in subsequent cycles. Both arms received Carfilzomib at a dose of 20 mg/m2 on days 1 and then 56 mg/m2 on days 8, 9, 15, and 16 of the first cycle, then 56 mg/m2 on days 1, 2, 8, 9, 15, and 16 in subsequent cycles, while 20 mg of Dexamethasone was administered on days 1, 2, 8, 9, 15, 16, 22, and 23 of each cycle.
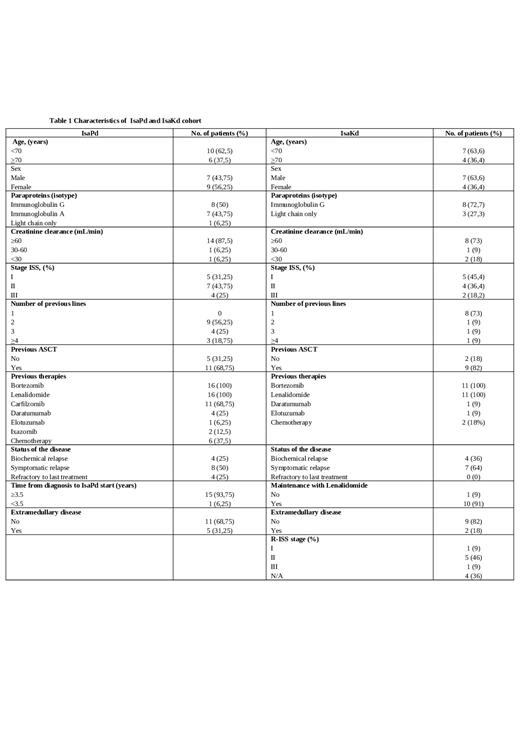
Results: The median age of the 16 IsaPd-treated patients was 67.5 years (range 48-89 years) and 61 years (range 49-84 years) for the IsaKd population; 44% and 64% were male, respectively (Table 1). The median number of prior therapies was 2 (range 2-4) for the IsaPd and 1 (range 1-4) for the IsaKd cohort; 11 (68.75%) and 9 (82%) received prior autologous stem cell transplantation (ASCT), respectively. Four patients in both populations started treatment because of biochemical relapse, and no refractory patients were recorded in the IsaKd cohort. Three patients (2 in the IsaPd population and 1 in the IsaKd cohort) had creatinine clearance ≤30 mL/min. Among the IsaKd-cohort, 5 (46%) had R-ISS 2 stage and three had a 17p deletion.
At the last data collection, 13 cases in the IsaPD population were evaluable for response. The median number of courses administered to date was 4 (range, 1-14). The overall response rate (ORR) was 69%, with 2 stringent complete remissions (sCR) (15%) and 1 very good partial remissions (VGPRs) (7.7%). The median time to first response was 1 month, while the median time to best response was 2 months. In the IsaKd population, 10 patients were evaluable with an overall response rate (ORR) of 100%, with 1 complete remission (sCR) (10%), 1 complete remission (10%), and 5 very good partial remissions (VGPRs) (50%). A median time to first response of 1 month was also recorded in this population, whereas the median time to best response was 3 months. Renal insufficiency and age > 70 years had no significant impact on the likelihood of achieving a response in either population.In the IsaPd cohort, after a median follow-up of 6 months (range 1-14 months), 9 patients discontinued treatment, 7 due to disease progression, 1 due to toxicity, and 1 due to patient choice) Six patients died (5 patients from progressive disease and 1 from secondary acute myeloid leukemia). Common grade 3 or 4 adverse events were fatigue (57%), thrombocytopenia (35%), neutropenia (57%), lymphocytopenia (15.9%), anemia (29%), and infection (21%). Infusion reactions occurred in only 2 patients (12.5%) and were grade 1 or 2 without treatment discontinuation. In the IsaKd population, the median follow-up time was 4 months (range 1-8 months). 1 patient discontinued treatment and died due to disease progression. Grade 3 or 4 adverse events were fatigue (18%), thrombocytopenia (18%), neutropenia (18%), anemia (36%), and infection (63%) No infusion reactions occurred.
Conclusions: Despite the limited number of cases, our preliminary real-world data confirm that Isa-based treatment is an effective and safe therapy for RRMM patients, broadly consistent with results from controlled clinical trials.
Disclosures
Fozza:Sanofi: Research Funding; Amgen: Research Funding; BMS: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal